Gases Practice Test - Answer Key
Back to the other Gases Practice Tests and other General Chemistry Practice Tests
Go To -> Practice Test - Answer Key
- A 4.40-g piece of solid CO2 (dry ice) is allowed to sublime in a balloon. The final volume of the balloon is 1.00L at 300 K. What is the pressure of the gas?
P = 24.6 atm
- The 2 main components in air are nitrogen and oxygen gas. Air is 79% N2 and 21% O2 by volume. Considering only N2 and O2 in air, calculate the density of air at 1.0 atm and 25oC.
density = 1.18 g/L
- At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. A 0.8765-g sample of impure sodium chlorate was heated until the production of oxygen gas ceased. The oxygen gas collected over water occupied 57.2 mL at a temperature of 22 oC and a pressure of 734 torr. Calculate the mass percent of NaClO3 in the original sample. (At 22 oC the vapor pressure of water is 19.8 torr)
18.0% NaClO3, by mass
- A compound has the empirical formula CH. At a temperature of 200oC and a pressure of 770 torr the gaseous compound has a density 2.04 g/L. What is the molecular formula?
C6H6
- Compare the following 4 gases all in 1.00 liter containers at 1.00 atm pressure and 25oC.
(a) He (b) CH4 (c) Cl2 (d) NH3 (e) All equal
- Which has the highest density?
Cl2
- Which has the highest number of molecules?
All equal.
- Which has the highest average velocity?
He
- Which has the smallest average kinetic energy (K.E.)?
All equal.
- A 3.5 L helium balloon at 780 torr, 25o C is cooled in a bath of liquid nitrogen (77 K). If the pressure is now 760 torr what will be the new volume of the balloon?
V = 0.93 L - True or False.
If the temperature of the cylinder is changed from 25oC to 50oC, the pressure inside the cylinder will double.
False.
- Consider the three flasks diagramed below. Assuming the connecting tubes have negligible volume, what is the partial pressure of each gas and the total pressure after all the stopcocks are opened?
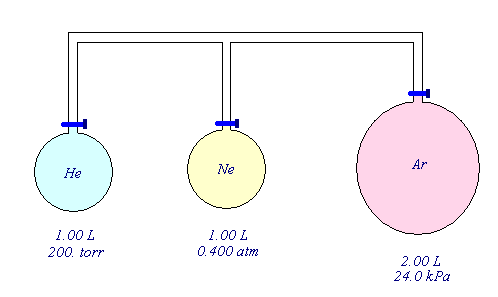
PHe = 0.0658 atm
PNe = 0.100 atm
PAr = 0.118 atm
Ptot = 0.284 atm
